You’re sitting in a cold exam room, and the words "clinical trial" finally come up. It sounds scary. Or maybe it sounds like a miracle. Honestly, it’s usually neither of those things. It's just a different way to get treated. People think a breast cancer clinical trial is a "last resort" for when everything else has failed, but that’s a massive misconception. In 2026, many of these trials are actually looking at how to reduce treatment—trying to figure out if we can do less surgery or less chemo while keeping the same survival rates.
Trial participation is how we got where we are today. Every single drug currently sitting in a pharmacy went through this. But when it's your life and your diagnosis, the "contribution to science" part feels secondary. You just want to know if it'll work.
The "Guinea Pig" Myth vs. Reality
Let's address the elephant in the room. No one wants to feel like a lab rat. But in a modern breast cancer clinical trial, you are arguably the most closely monitored patient in the hospital. You have a whole research team—nurses, coordinators, oncologists—watching your blood work and side effects like hawks.
There's this fear of the placebo. Let’s be clear: in cancer research, you are almost never given "nothing." That would be unethical. If a trial is testing a new immunotherapy, they usually compare the "Standard of Care" (what you’d get anyway) against the "Standard of Care PLUS the new drug." You’re getting the best known treatment as a baseline. Sometimes, if the trial is a "head-to-head" study, they’re comparing a new drug against an old one to see which is better. You aren't being left unprotected.
Wait, there’s a catch.
Randomization is real. If the trial is randomized, a computer decides which group you’re in. You can’t choose. Your doctor can’t choose. That lack of control is what freaks people out the most.
Why 2026 is a Pivot Point for Breast Cancer Research
The landscape has shifted. We aren't just talking about "breast cancer" anymore as one big category. We're talking about micro-niches. Triple-negative (TNBC), HER2-positive, HR-positive/HER2-low.
A few years ago, the DESTINY-Breast04 trial changed everything by proving that "HER2-low" patients could benefit from Enhertu. Before that, those patients were just stuck in the "HER2-negative" bucket. Now, a breast cancer clinical trial is more likely to be a "basket trial" or an "umbrella trial."
- Umbrella trials look at one type of cancer (like breast) but test many different drugs based on the specific mutations in your tumor.
- Basket trials test one drug across many different cancers that share the same mutation.
It’s hyper-personalized. If you have a PIK3CA mutation or a BRCA1/2 variant, there is likely a trial specifically targeting that biological "glitch." This isn't a shotgun approach; it's a sniper rifle.
🔗 Read more: Understanding BD Veritor Covid Test Results: What the Lines Actually Mean
The Financial Side Nobody Talks About
Medical care is expensive. Ridiculously so. One surprising "pro" of joining a breast cancer clinical trial is that the trial sponsor (usually a pharma company or a grant) often pays for the study drug and the extra tests.
However, don't assume everything is free.
Your regular insurance is still billed for "routine costs." This includes things you would have had anyway, like your regular doctor visits or standard scans. It’s a bit of a paperwork nightmare. You have to talk to the financial coordinator. Ask them specifically: "Who pays for the extra EKGs?" or "What happens if I have a side effect and need to be hospitalized?" Get it in writing.
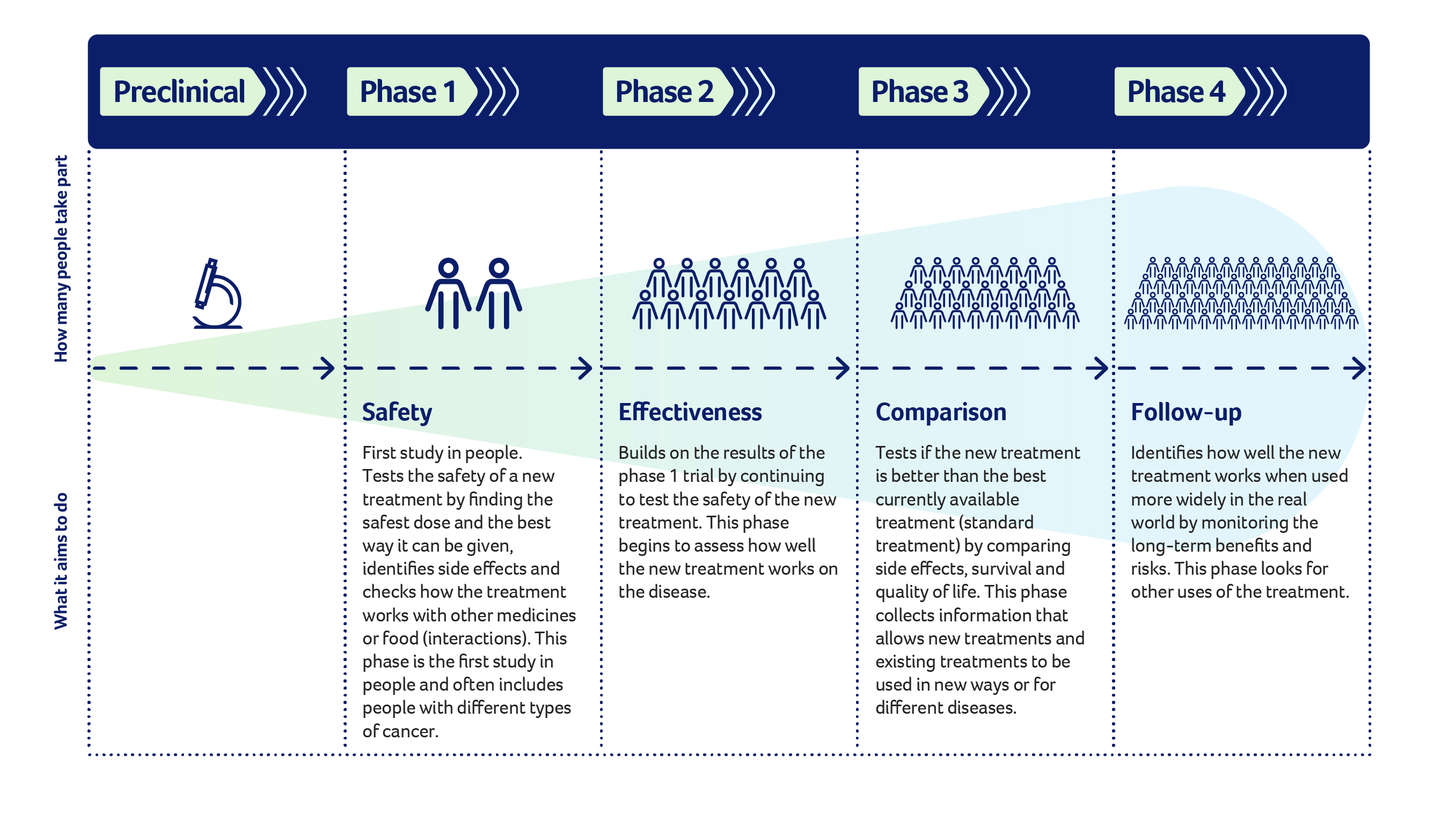
Different Phases: Which One Are You Looking At?
Not all trials are equal.
Phase 1 is the Wild West. They are looking for the right dose. They want to see if the drug is safe. It’s usually a small group of people. If you’re here, you’re likely looking for a breakthrough because standard treatments haven't done the job.
Phase 2 starts looking at efficacy. Does it actually shrink the tumor?
Phase 3 is the big one. This involves hundreds or thousands of people. This is where they prove the new treatment is better than what we already have. If you join a Phase 3 breast cancer clinical trial, you are often getting a drug that is very close to being FDA approved.
The "De-escalation" Trend
This is arguably the most exciting part of research right now. We spent decades hitting breast cancer with everything but the kitchen sink. Now, researchers are asking: "Can we do less?"
💡 You might also like: Thinking of a bleaching kit for anus? What you actually need to know before buying
The CompassHER2 trials and the DECRESCENDO study are great examples. They're looking at whether patients with certain HER2-positive profiles can skip the harsh chemotherapy and just take targeted antibodies if they show a good early response. Imagine keeping your hair and avoiding the "chemo brain" because a trial proved you didn't actually need the toxic stuff. That’s a massive win for quality of life.
But it’s a gamble. If you do less, is there a higher chance of recurrence? That’s what the trial is trying to answer. You have to weigh the desire for a "gentler" treatment against the security of the "aggressive" standard.
Inclusion and Exclusion: Why You Might Be Rejected
It’s incredibly frustrating. You find a trial, you're excited, and then you’re told you don't qualify.
Maybe your heart function isn't "perfect" enough. Maybe you had another type of cancer five years ago. Maybe your white blood cell count is just slightly off the required mark. These "Inclusion/Exclusion Criteria" are strict because researchers need a "clean" data set to prove the drug works. It feels personal, but it isn't. It’s about the statistics.
If you get rejected from one breast cancer clinical trial, don't stop. There are thousands. Databases like ClinicalTrials.gov are a mess to navigate, but platforms like BreastCancerTrials.org or the Metastatic Breast Cancer Alliance make it way easier to filter by your specific subtype.
Real Talk: The Side Effects
Don't let anyone tell you trial drugs are "safer" just because they're "targeted." Targeted doesn't mean "no side effects."
Immunotherapies can cause your immune system to attack your own organs (colitis, pneumonitis). Antibody-drug conjugates (ADCs) can cause low blood counts or lung inflammation. You are essentially a pioneer. You might experience a side effect that hasn't been widely documented yet.
You have to be okay with that uncertainty. You have to be okay with more frequent blood draws. More biopsies. More time spent in the waiting room.
📖 Related: The Back Support Seat Cushion for Office Chair: Why Your Spine Still Aches
How to Actually Join a Trial
You don't just sign up online like a newsletter. It's a process.
- The Screening: You'll have blood work, scans, and maybe a new biopsy to confirm you meet the criteria.
- Informed Consent: This is a long document. Read it. It lists every scary thing that could happen. It’s not meant to scare you off; it’s meant to be honest.
- The Baseline: They need a "before" picture of your health.
- Treatment: You start the regimen.
You can leave whenever you want. People forget that. You are not a prisoner. If the side effects are too much, or if you just change your mind, you can walk away. The "Informed Consent" is an ongoing process, not a one-time signature.
Actionable Steps for Patients and Caregivers
If you're considering a breast cancer clinical trial, stop waiting for your oncologist to bring it up. Most doctors are busy. They might default to the standard treatment because it's easier and proven. You have to be your own advocate here.
Ask these specific questions at your next appointment:
- Is there a trial for my specific subtype (e.g., Triple Negative, HER2-low) at this hospital?
- If not here, is there one at a nearby NCI-Designated Cancer Center?
- How will this trial change my daily life? (e.g., "Will I be here every week?")
- What happens if the treatment doesn't work? What's the "exit plan"?
- Are there travel reimbursements? (Some trials pay for your gas or hotels).
Use the right tools:
Go to the Breast Cancer Research Foundation (BCRF) website or the Susan G. Komen clinical trial finder. Don't just browse; use the filters for your stage and location.
Get a second opinion:
Major academic centers (like Dana-Farber, MD Anderson, or Memorial Sloan Kettering) often have trials that smaller community hospitals don't. A one-time consultation at a big research hub can open doors you didn't even know existed.
The goal isn't just to "survive." The goal is to thrive with the best possible science on your side. Sometimes that science is already at the pharmacy, and sometimes it’s still in a trial. Both are valid paths. Just make sure you’re choosing the one that fits your life, not just your diagnosis.