Ever stared at a weather report or a scuba diving gauge and wondered why the heck we use so many different units for the exact same thing? It’s annoying. One minute you’re looking at kilopascals, the next it’s pounds per square inch, and then—boom—you’re hit with atmospheres and millimeters of mercury. If you just need the quick answer: 1 atm to mmhg is exactly 760 mmHg.
That's the magic number.
But why 760? It feels arbitrary, doesn't it? It’s not a round number like 100 or 1,000. To understand why this specific conversion matters, you have to look at a guy named Evangelista Torricelli. Back in the 1640s, people didn't even think air had weight. They thought it was just... nothingness. Torricelli, a student of Galileo, proved them wrong by filling a long glass tube with mercury, flipping it upside down into a dish, and watching what happened. The mercury didn't all spill out. It stopped at a specific height.
That height was about 760 millimeters.
The Physics Behind 1 atm to mmhg
Think about the air above your head right now. It stretches up for miles. Even though it feels like nothing, all those gas molecules are being pulled down by gravity. They’re pressing on you. At sea level, that "column" of air exerts a specific amount of force. When Torricelli did his experiment, he realized the air pushing down on the mercury in the bowl was perfectly balanced by the weight of the mercury inside the tube.
At standard sea level, that balance point happens at 760 mm.
Hence, 1 standard atmosphere (atm) equals 760 millimeters of mercury (mmHg). This isn't just some dusty history lesson; it's the foundation of how we measure blood pressure, vacuum seal food, and keep airplanes from falling out of the sky.
If you're doing chemistry homework or calibrating a lab sensor, you’ll also see the term Torr. For almost all practical purposes, 1 mmHg is 1 Torr. They are named after Torricelli, obviously. Technically, there’s a tiny, microscopic difference based on modern definitions of the Pascal, but unless you’re working for NASA or CERN, you can treat them as identical.
Why Mercury?
You might wonder why we don't use water. It's cheaper. It's everywhere. Well, physics has a funny way of making things difficult. Mercury is incredibly dense—about 13.5 times denser than water. If you tried to build a barometer using water to measure 1 atm, your glass tube would have to be over 33 feet high. That’s a three-story building just to check if a storm is coming.
Mercury keeps the equipment small.
🔗 Read more: White Pages Opt Out: Why Your Personal Info is Still Stuck There and How to Fix It
Even though we’re moving away from liquid mercury because, you know, it’s toxic, the unit "mmHg" has stuck around like a stubborn relative. Doctors still use it for blood pressure. When a nurse says your pressure is "120 over 80," they mean your heart is pushing blood with a force equivalent to 120 millimeters of mercury. Imagine a tiny silver column rising in a tube; that’s the literal measurement of your pulse.
Converting 1 atm to mmhg in the Real World
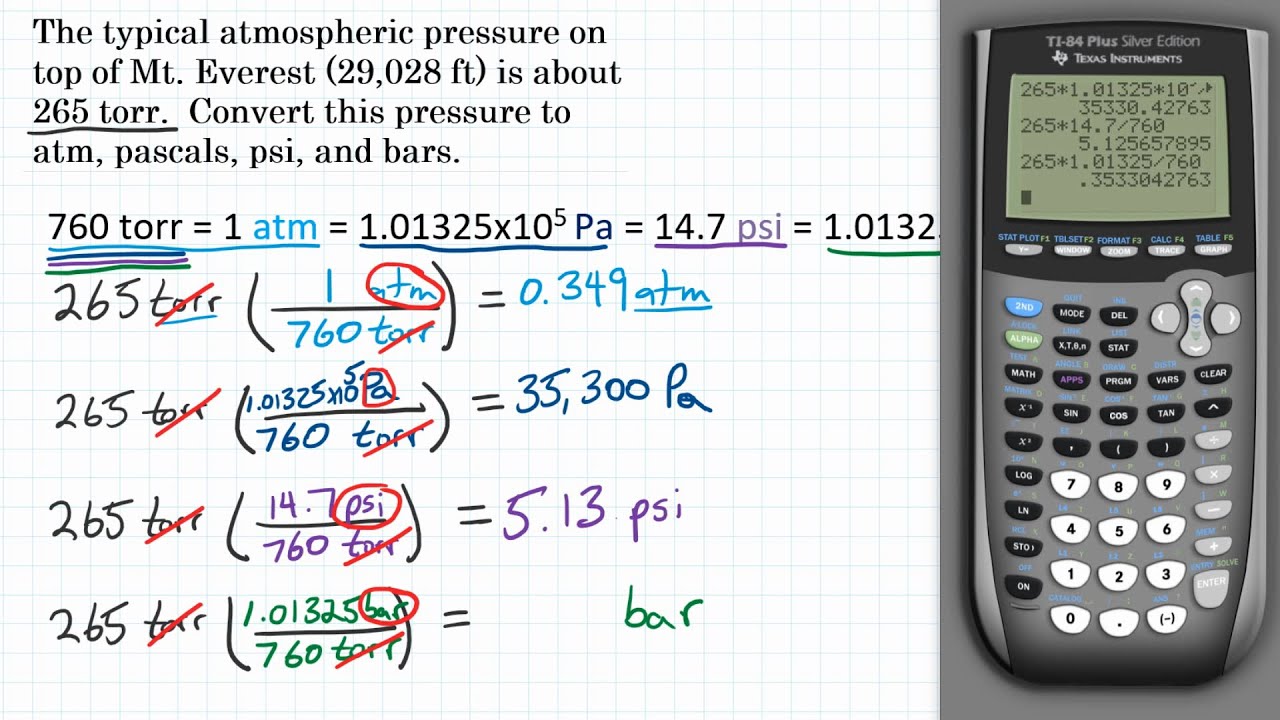
Let's look at how this plays out if you aren't sitting at sea level. If you climb Mount Everest, the atmosphere is "thinner." There’s less air above you, so there's less pressure. At the summit, the atmospheric pressure drops to about 250 mmHg. That’s roughly 0.33 atm.
Your lungs are used to 1 atm. At 0.33 atm, they can't grab enough oxygen.
| Pressure Unit | Value relative to 1 atm |
|---|---|
| Atmospheres (atm) | 1 |
| Millimeters of Mercury (mmHg) | 760 |
| Torr | 760 |
| Pounds per Square Inch (psi) | 14.7 |
| Kilopascals (kPa) | 101.325 |
You see that 101.325 kPa? That’s the "modern" metric version. Most of the world uses it for weather. But in the US and in many specialized scientific fields, the 1 atm to mmhg relationship remains the gold standard because it’s based on a physical reality you can actually see in a tube.
Common Mistakes People Make
Most people mess up the math because they try to overcomplicate it. If you have 2 atm, you just double 760. 1,520 mmHg. Easy. If you have 0.5 atm, it’s 380 mmHg.
The real trouble starts when you mix up "Standard Atmosphere" and "Technical Atmosphere." A technical atmosphere (at) is slightly different, based on one kilogram of force per square centimeter. It's about 735.5 mmHg. If you’re an engineer working on old European machinery, this distinction is huge. If you use the wrong "atmosphere," your pressure readings will be off by about 3%, which is enough to blow a seal or ruin a batch of chemicals.
Also, don't confuse mmHg with inHg (inches of mercury). Aviation in the United States uses inches of mercury for altimeter settings. 1 atm is about 29.92 inHg. If a pilot gets these mixed up, they might think they’re at 30,000 feet when they’re actually much lower.
The Future of Pressure Units
Are we ever going to stop using mmHg? Honestly, probably not.
Medical schools are deeply entrenched in it. There have been pushes to move blood pressure readings to kilopascals, but it hasn't gained much traction. It’s a bit like the imperial vs. metric debate in the US. When an entire global industry is trained on one specific number, changing it creates more risk than it’s worth.
We see this in high-vacuum physics too. When scientists are trying to create a vacuum—like inside a particle accelerator—they measure how much "stuff" is left in the chamber. They don't use atm because the numbers would be way too small (like 0.0000000001 atm). Instead, they use Torr or mmHg to describe those tiny, tiny pressures.
Actionable Steps for Conversion
If you're working on a project that requires switching between these units, stop trying to do the long-form division in your head.
- Verify your base: Ensure you are starting with a "Standard Atmosphere" (atm) and not a "Technical Atmosphere" or "Bar."
- The Constant: Memorize 760. It is the only number that matters for this specific conversion.
- Check your Direction: - To go from atm to mmHg: Multiply by 760.
- To go from mmHg to atm: Divide by 760.
- Use a Calibration Tool: If you are working in a lab setting, use a digital manometer that allows for unit switching to avoid manual calculation errors.
- Context Matters: If you’re reading a weather report, look for "hPa" or "inHg." If you’re looking at a gas canister, look for "psi" or "bar." If you’re looking at a physiology textbook, it’s almost always going to be mmHg.
Understanding that 1 atm to mmhg is 760 isn't just about passing a test. It's about understanding the weight of the world around you. Every square inch of your body is currently being pressed on by nearly 15 pounds of air. You don't feel it because your internal pressure is pushing back with the exact same force. We are all walking barometers, perfectly balanced at 760 mmHg.